At drugis.org it is our mission to develop information technology to assist and improve how decisions regarding the use of medicines are made. Our main goals are transparency and efficiency. We aim to deliver valuable working software which is continuously improved based on feedback from users and researchers. As part of the GetReal Initiative, we partner with industry, regulators, and academics.
Motivation
Decisions concerning the favourable and unfavourable effects of medicines often rely entirely on the expert judgment of those responsible. Reliance on such subjective assessment hides the reasoning behind the decision and causes the process to be insufficiently transparent and traceable. We aim to build a decision support system that combines proven statistical methods and quantitative decision modeling with a structured database of evidence from clinical research. Such a system enhances the transparency of decisions by clearly separating the (objective) evidence from the (subjective) trade-off judgments inherent in the decision.
Health policy decisions regarding alternative treatment options are often informed by systematic reviews of clinical trials. Typically only the end product of systematic reviewing (a report summarizing the evidence), is made widely available. However, access to the intermediate results of literature searching, publication screening, and data extraction could greatly enhance the efficiency of future reviews. Given the increasing scope of systematic reviews, this uneccessary duplication of effort must be eliminated. This requires a change in the current culture of data protectionism, and suitable software that enables convenient and useful sharing of the intermediate results.
Approach
Our previous ADDIS 1.x prototype showed the usefulness of a benefit-risk decision support system for local use. It enables network meta-analysis and multiple criteria decision analysis based on a structured database of clinical trials data. We have now re-implemented the system as a web platform to ease collaboration. The shareable study data repository also increases the efficiency of any further systematic reviews on subjects that already have an ADDIS data set.
The Problem
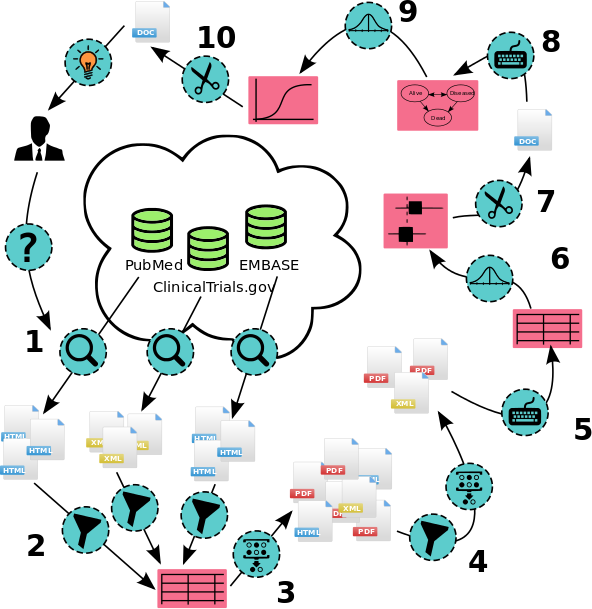
Legend
- Query of multiple databases, yielding results in different formats
- Title/abstract screening; enter results in spreadsheet or database
- De-duplication of included abstracts, retrieve full texts
- Full text screening, identify duplicate publications of same trial
- Manual data extraction; enter results in meta-analysis software
- Statistical analysis / evidence synthesis
- Reporting of evidence synthesis results; potential publication
- Enter relevant evidence into cost-effectiveness model
- Cost-effectiveness analysis
- Reporting of cost-effectiveness analysis for decision maker or publication
Health policy decision making is often informed by a systematic review, followed by a statistical analysis and decision modeling. These steps are performed using an ad-hoc series of disconnected software tools. The rapidly expanding scope of health policy decision making and volume available data increases the costs of systematic reviews, which had already reached the thousands of person-hours over a decade ago.
Systematic reviews are thus costly to perform, there is very little return on investment for sharing data and meta-data collected in the process, and sharing data early on has the associated risks of scooping and perhaps unwanted scrutiny. Moreover, sharing is difficult because the data and meta-data are fragmented between a number of different tools that use different and incompatible formats. This has led to a culture where researchers are reluctant to share their intermediate results, and work is often duplicated between different systematic reviews.
The Solution
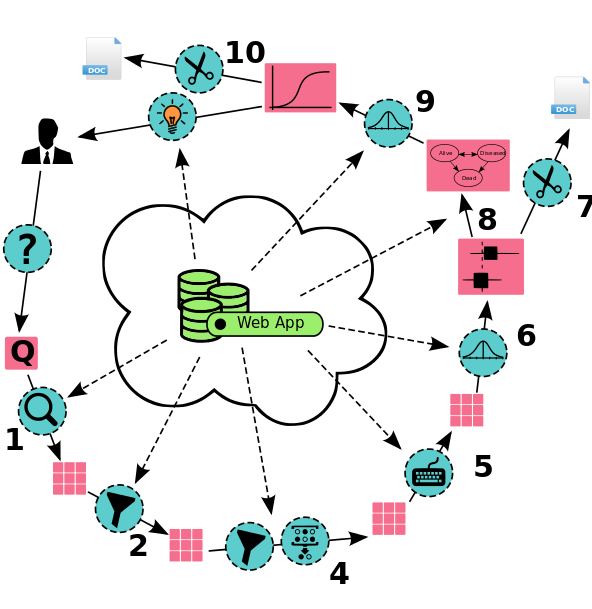
Legend
- Automatic query of multiple databases returning linked data
- Assisted title/abstract screening
- N/A (abstracts are automatically de-duplicated)
- Assisted full text screening, identify duplicate publications of same trial
- Assisted data extraction
- Statistical analysis / evidence synthesis
- Reporting of evidence synthesis results; potential publication
- Automatic transfer of evidence to cost-effectiveness model
- Cost-effectiveness analysis
- Reporting of cost-effectiveness analysis; potential publication
The process can be made more efficient and reproducible by an integrated software toolchain that captures the entire workflow. Such a system would provide several incentives that make sharing of intermediate results more attractive. First, it would remove the technical barriers to sharing data by capturing all intermediate results in a single repository, and sharing of data could be the default setting. Second, it would simply provide a better tool chain for systematic review and decision support by automating the transfer of data between steps and assisting labour intensive steps such as literature screening. Finally, the investment of performing a systematic review can be reduced by re-using what others have shared. This would decrease the time-to-publication or time-to-decision and enhance the cost-effectiveness of evidence-based decision making.
Conclusion
Systematic reviewing is currently a laborious process that is mainly performed "offline" using an ad-hoc collection of disconnected tools. This makes the sharing and re-use of intermediate results nearly impossible, leading to duplication of effort and an adversarial research environment. Existing information technologies could be leveraged to build a more integrated system to support systematic reviewing and preserve intermediate results. Such a system can create a virtuous cycle in which previously shared results make sharing an ever more attractive option. Eventually, this will decrease the cost of systematic reviewing drastically, which in turn enables evidence-based decision making to be applied more often and more rigorously.
Our Commitment: Open Development
We are committed to develop our software 'in the open'. This means that we publish our software as early as possible and as often as possible, enabling others to use and critique our software and development process. On this website, we will keep you up to date on the current status of our software and new releases. Furthermore, all source code is made publicly available, under an Open Source license, allowing deep peer-review of our software. Our liberal software licensing also allows others to create spin-off programs from our source code, as long as they likewise make their source code available.